MitraVerse
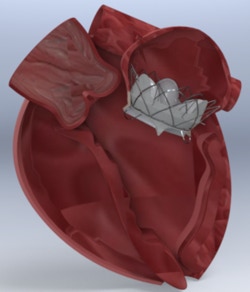
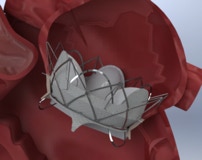
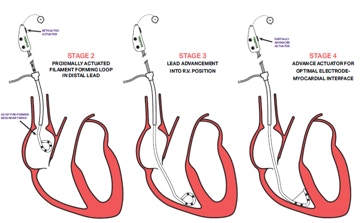
Is developing a novel two component mitral valve replacement solution designed from day one to be delivered transseptal and not obstruct the left ventricle outflow tract.
Unique features our device has over the competition:
1) Maintain native leaflet function until second component (valve) is deployed into the Docking Station 2) Our design minimizes LVOT and thrombus allowing us to expand the patient population of use 3) The two component system is smaller than 22Fr and since our two components do not require clocking or complicated delivery methods it is easier to deploy
Development Status
We are in the process of completing our design dossier allowing us to move rapidly toward developing and de-risking the design oncer our first round of financing is complete
Intellectual Property
Patent applications filed
Funding Status
We are in the process of seeking investors to finance our development efforts through FDA submission and our First-in-Human clinical study.
Financing and Timeline:
$12 million / 48 months to complete FDA submission and First-in-Human study
For investment inquires please contact